Genetics and Fertility Video Series
Chapter 1 - Introduction to Genetics
Chapter 2 - Inheritance and Mutations
Chapter 4 - Results and Risks
Chapter 6 - Additional Information
Transcripts
These videos were created to inform patients about genetics and genetic testing in conjunction with infertility and in vitro fertilization treatment.
You’re watching Introduction to Genetics, chapter one of the genetics and fertility video series from the American Society for Reproductive Medicine.
Genetics is the study of how traits and characteristics are passed from parents to their children. Genetic testing helps us understand who is at risk for passing on or receiving those traits. This video will talk about basic genetic biology, how traits are passed and inherited, and common types of genetic testing.
Let’s start with some basic terms.
Cells are the basic building block of all living things.The basic parts of cells are:
- Cell membrane—this is the outer wall of the cell that holds in all the other parts.
- The cytoplasm is the area between the nucleus and cell membrane.
- Cytoplasm contains mitochondria, which make energy for the cell.
- The nucleus is the command center of the cell and holds the blueprints for how the cell functions.
- Inside the nucleus are chromosomes which are made up of genes, which in turn are made up of deoxyribonucleic acid—better known as DNA. DNA is arranged in a specific sequence or order and it is this order that tells the cells how to function.
Wait what?
Mutated genes?Mutations are changes in how the DNA is arranged in the gene represented here by the color purple (B).

Everyone has some mutated genes and, a lot of the time, they don’t affect us in a negative way. Sometimes though, abnormal genes can show up as a serious medical condition, a genetic disorder, or cause a miscarriage.
Since we all have gene mutations, it’s often when two people with mutations in the same gene have children together that a problem occurs.
Before we talk about why a mutation might be important, let’s first talk about inheritance of mutations and disease: who gets what, and how.
You’re watching Inheritance and Mutations, chapter two of the genetics and fertility video series from the American Society for Reproductive Medicine.
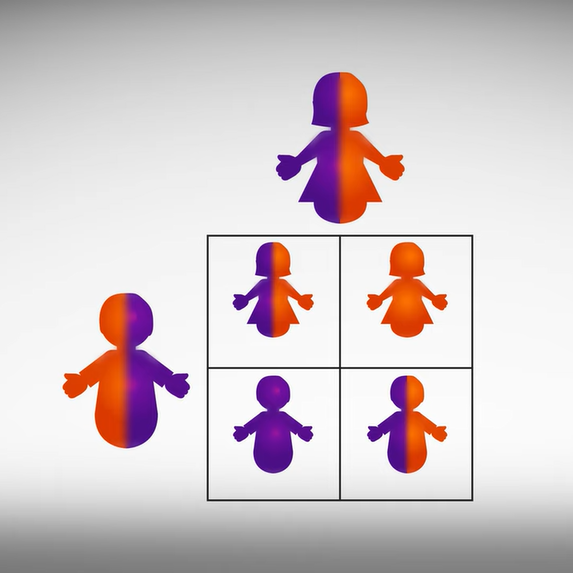
Diseases can be passed down in different ways, depending on the disease and how many copies of the gene a child inherits. When illustrating inheritance it helps to use a Punnett square. The square is named for Reginald C. Punnett, who devised the following approach.
Draw a large square divided into four smaller squares, you should have two rows and two columns. One parent is drawn on the top and one parent is drawn on the side, shown here in gray.

The diagram is used to predict the genetic makeup of the children, also shown here in gray. In our example each square represents 25% of the genes a child will inherit for a particular trait for any child born from the two parents. Each parent can pass down one of two potential chromosomes. For instance the mother in this example scenario has one chromosome with a mutation in purple, and one chromosome without a mutation in orange. The mother passes one copy of each of her chromosomes down each column from top to bottom. Meanwhile the father who has one chromosome with a mutation in purple and one chromosome without a mutation in orange passes each of his chromosomes across each row from left to right.
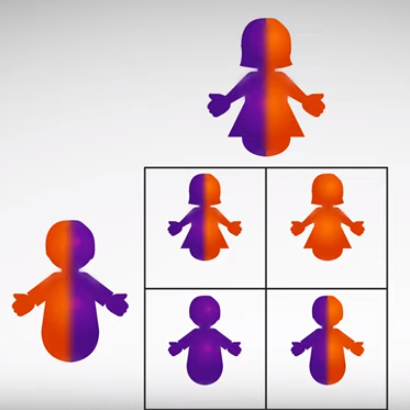
Each child has a 50% chance to receive one of two chromosomes from their father and a 50% chance to receive one of two chromosomes from their mother. Typically a child will not receive two chromosomes from one parent, and typically a child will not have more than two of each chromosome.
So the outcome for this scenario means that there is a 25% chance that one child will be born with two chromosomes that carry the mutation, and a 25% chance that the child will be born without the mutation on either chromosome. The remaining 50% means that the child will be born with one copy of the mutation and one copy of the chromosome without the mutation like their parents.
The Punnett square determines the likelihood of having an affected child with each pregnancy. This likelihood resets for each pregnancy, so even if your child is affected, their siblings will have the same chance of having a mutation or not having a mutation. So while it is rare for two parents with one mutation each to have four children that all have two copies of the mutation it is still possible. Now that we understand the basics of inheritance let’s look as some of the different types of genetic mutations.
Genetic disorders caused by mutations in a single gene are called monogenic. These disorders can be autosomal—meaning the mutation is on any of the autosomes shown here in orange—or sex-linked—meaning the mutation is on the X or Y chromosome, shown here in pink and blue.

Autosomal mutations can be either recessive or dominant.
Autosomal recessive means that you need 2 copies of the mutated gene to have the disease. Examples are sickle cell anemia and cystic fibrosis. If you only have one copy of a gene with an autosomal recessive disease mutation, and your other copy is normal, you are called a carrier. This means you won’t have the disease yourself but could pass it on to your children if the child’s other parent is a carrier and has a recessive mutation in the same gene.
The chance of each outcome breaks down like this:
- Affected person plus a non-carrier = All children are carriers, no children have the disease, and no children are without the mutation.
- Affected person plus a carrier = Statistically, half the children will have the disease and half will be carriers.
- Carrier plus a carrier = Statistically, half the children will be carriers, 1/4 will have the disease, and 1/4 will not have the mutation.
- Carrier plus a non-carrier = Statistically, half of children are carriers and half are non-carriers.
It breaks down like this:
- If one parent has one mutated copy and the other parent is healthy, there‘s a 50% chance of having a healthy child and a 50% chance of having an affected child with one mutated copy.
- If at least one parent has both copies of an affected gene, all children will be affected- this is rare.
- If both parents have one mutated copy of the gene, there is a 1 in 4 chance of a healthy child, a 2 in 4 chance of getting a child with one mutated copy (and thus affected), and a 1 in 4 chance of having a child with 2 mutated copies. Because this is autosomal dominant, the overall chance that a child will be affected by the disease is 3 in 4.
Mutations can also occur in the sex chromosomes
X and Y.
These mutations can be trickier because, depending on the sex, the chromosome pairs are not the same. Men are XY, women are XX.
Some Y-chromosome mutations can cause absent or low sperm counts. These mutations can be passed down from fathers to sons.
Like autosomal mutations, X-linked diseases can be recessive or dominant. This is important, because in the case of X-linked recessive conditions, such as hemophilia, the chance of a child having the disease is different for boys and girls.
The chance of having an affected child with an X-linked recessive disease breaks down like this:
- Carrier mother and healthy father: There is an equal chance of having a healthy girl, a healthy boy, an affected boy, or carrier girl. Statistically, this means that half of the children, boys and girls together, will be unaffected. For the other half of the children, the girls will be carriers and the boys will be affected.
- Non-carrier healthy mother and affected father: All the girls will be carriers since the father can only pass his mutated X chromosome to the girls, while none of the boys will have the disease since the father passes his Y chromosome.
- An affected mother with 2 copies, and affected father with one copy: All children will be affected. This is rare.
- Carrier mother with one copy and an affected father: Half of the girls will be carriers and half of the girls will be affected. Half of the boys will be affected and half of the boys will be unaffected.
You’re watching Genetic Testing, chapter three of the genetics and fertility video series from the American Society for Reproductive Medicine.
Now that we’ve gone over what inherited mutations are and how they can be passed from parent to child, let’s talk about how to test for them. The first choice is who or what to test.
- the people who want to have a baby,
- the embryos before they are transferred, or
- the fetus after a woman gets pregnant.
Each test has its own advantages and place in reproductive planning. (Let’s explore the kinds of testing available, and where they may be suitable.)
Parental testing:
Testing the potential parents can help a couple tell whether additional genetic testing will be needed.
If a member of the couple has a family history of genetic disease or is a known carrier of a genetic disease, genetic testing may be indicated for the other parent.
If neither parent carries a particular gene mutation, the chances of having an affected child are very low. The couple can choose to conceive naturally and forgo testing of the embryo.
An advantage of testing the parents is that the results tell you the chances of having an affected child, however, this testing does not tell you whether the child is actually affected.
If both potential parents have an autosomal recessive mutation, they may choose to undergo in vitro fertilization or IVF, then biopsy the resulting embryos, and freeze and test each biopsy. Later, only embryos that have tested normal will be transferred generally in a frozen embryo cycle.
If one parent has has an autosomal recessive mutation and the other doesn't, the medical team can help evaluate the risk of having an affected child and the couple can make an informed decision. However, testing of the embryo in this circumstance is extremely unlikely to be helpful.
Embryo Testing:
If parents have known gene mutations for a particular genetic disease, preimplantation genetic testing, PGT, could be considered. This involves testing the individual embryos by removing cells with a biopsy in the IVF laboratory, which are then sent for genetic analysis. This gives information about the specific mutations of that particular embryo before there is a pregnancy. This allows the parents to choose to transfer only embryos that don't have a mutation or combination of mutations that cause the particular genetic disease.
The disadvantage here is that the embryo testing requires IVF even if there is no infertility, which can be expensive and invasive. In some cases the result of the test may not be conclusive for every embryo.
Fetal testing:
Testing the fetus at risk for a genetic disease during pregnancy gives more accurate information than testing the embryo before a pregnancy begins. The cells that are tested come from the tissues and fluid around the fetus that contains the fetus’s DNA. Testing the fetus at risk for a genetic disease during pregnancy gives more accurate information than testing the embryo before a pregnancy begins, because there are more cells available for analysis. This strategy also can allow couples to avoid IVF and get pregnant on their own.
The disadvantage here is that if a pregnancy is ongoing, a couple may have to choose between having an affected child or terminating the pregnancy, which can be emotionally difficult.
So, how do you decide whether to test the fetus, your embryos, or you?
Several organizations of experts have made recommendations about who should be screened for what. The guidance is based on your family history, ethnicity, or what is a common genetic mutation that occurs in the region where you live. For instance, in the US, everyone should be offered testing for cystic fibrosis and spinal muscular atrophy because these are two of the most common autosomal recessive conditions seen in US children.
Ashkenazi Jews are at much higher risk for Tay Sachs or Canavan disease, so people of Ashkenazi descent should be offered screening for those diseases and several others. As with other risk groups there is a common set of mutations in the Ashkenazi Jewish population. Manufacturerers of genetic tests often bundle a group of tests together into a single panel rather than offering individual tests for each mutation. The individual mutations that each panel tests for varies from manufacturer to manufacturer.
People of African, Asian and Mediterranean heritage should be offered screening for certain blood disorders, like sickle cell disease or thalassemia. You can visit the websites of the CDC, ACOG, ACMG and specialty organizations for the latest recommendations or discuss with your healthcare provider.
Expanded Carrier Screening
Since many of us are of mixed ethnic background, it can be difficult to know what to test for. Expanded carrier screening is one of the options to help with this—just one test allows a person to be screened for up to several hundred disorders.
A disadvantage here is that the results can be hard to interpret. A positive test result tells you whether you carry the trait, but severity and treatment can vary from disease to disease. Also, no one test covers all mutations, so you could carry a condition that wasn’t tested for. Expanded carrier screening is new technology and new tests are being developed all the time. The accuracy of these tests hasn’t been verified independently other than by the manufacturer. These tests can also be expensive and often aren’t covered by insurance.
If you’re considering expanded carrier screening, make sure you have an expert, like a geneticist or genetic counselor, to help you understand your results and check with your insurance carrier about your coverage.
Now, let’s talk about preimplantation genetic testing of embryos
A biopsy is performed in the IVF laboratory, where cells are taken from the embryo and tested. This requires IVF so that the embryos are available for testing, and allows for decisions to be made on the results of the test.
There are two basic types of testing: PGT-A, aneuploidy screening, and testing for specific inherited gene or chromosome changes called PGT-M and PGT-SR
Preimplantation genetic testing that looks for specific specific inherited genetic changes include PGT-M and PGT-SR. PGT-M checks for single gene mutations and PGT-SR is used to detect unbalanced chromosome rearrangements. Remember that a normal or euploid human embryo has 46 chromosomes—23 pairs, one copy of each pair comes from each parent, including the numbered chromosomes one to twenty two and the sex chromosomes X and Y.
PGT-A, which checks for extra or missing chromosomes called aneuploidy can find conditions like Down syndrome—an extra chromosome number 21. However aneuploidy can occur with any of the chromosome pairs. Most cases of aneuploidy result in failed embryo transfers or miscarriages. Therefore, the biggest advantage to PGT-A is to identify embryos with a higher likelihood of resulting in a successful and healthy pregnancy.
Although aneuploidy is a potential testing result it isn't an inherited genetic trait, but is a instead related to the physical condition and quality of the biological mother’s eggs. Aneuploidy is linked to age. By the time a woman is 35 years old, about half her embryos are expected to be aneuploid. By age 40, this increases to about 80%.
What About A Karyotype?
A karyotype is what most people think of when genetic testing is mentioned. It’s a picture of the actual chromosomes arranged in pairs. It can show structural abnormalities on the chromosomes and counts the number of copies of chromosomes.
Because a karyotype is a big-picture view of how the chromosomes are organized, it can’t tell us anything about gene mutations that might be there. It also can’t detect tiny structural errors on individual chromosomes. These could be microdeletions—tiny missing segments of DNA—or microduplications—tiny extra copies of DNA that can lead to disease/health problems.
PGT-A is different from a karyotype in that PGT-A doesn’t provide the image of the chromosomes because there aren’t enough cells to test. Instead, PGT-A analyzes pieces of a chromosome and infers the number and presence of the chromosomes from the pieces that are there.
You’re watching Results and Risks, chapter four of the genetics and fertility video series from the American Society for Reproductive Medicine.
The preimplantation biopsy procedure itself doesn’t introduce any genetic errors. Most embryos are biopsied about 5 to 6 days of development. By this time, the cells can be taken from the outer layer that will become the placenta. The embryo may be frozen at this point to allow time for the testing.
It’s reassuring to know that, for the most part, a few cells can be removed safely from an embryo. In fact, embryos also lose cells in other situations, like when an embryo splits into identical twins. Sometimes cells are lost during the freeze and thaw of a frozen embryo but a healthy baby is born. Though no procedure, including biopsy, is completely risk-free, the benefit of the information gained can balance that risk. Sometimes, embryos stop dividing after biopsy, but it’s hard to know if this is because of the procedure or if it would have happened anyway.
Other risks to the embryo are small. For instance, though the embryo stays at your original lab, the biopsied cells are often sent to a different lab for testing. There is a chance that those cells could be lost or damaged in transit to the testing facility. There’s also the possibility that the sample could be contaminated. Laboratories have protocols and safeguards to minimize the risks. Another risk is that in testing only a few cells from the part of the embryo that becomes the placenta, there is a small chance that they don’t represent the rest of the cells in the embryo.
We can’t test every cell and then still transfer the embryo, so this may be the best test available to help choose the best embryo for transfer. This is why we still recommend considering genetic testing during pregnancy. There is a small chance that results from your embryo's testing may be different than results obtained during pregnancy, even after transfer of a tested embryo.
Also, we don’t think that removing those few cells damages the embryo, but it is possible to cause harm to the embryo or have an effect that we don’t yet understand. So far, children born from this process seem healthy. Even with PGT, other testing during pregnancy could reveal genetic issues unrelated to what was screened for with PGT.
It’s possible, though not likely, to discard an embryo that tested abnormal but was truly normal, or transfer an embryo that tested normal but was truly abnormal. Also the significance of some test results are not well understood. There is still much that science can’t explain about this process.
It can be disappointing if your cycle results in no normal embryos to transfer. But this could be helpful information. You can try another cycle to identify a normal embryo sooner than if untested embryos were transferred that resulted in miscarriages or negative pregnancy tests.
You’re watching Results Summary, chapter five of the genetics and fertility video series from the American Society for Reproductive Medicine.
HOW ARE TEST RESULTS REPORTED?
For PTG-M, there are 5 possible test results for each embryo:- unaffected,
- unaffected carrier,
- affected,
- inconclusive, and
- no result.
- euploid,
- aneuploid,
- inconclusive,
- no result, or
- mosaic.
- embryo transfer,
- retest, and
- discard.
WHAT IF ALL EMBRYOS TEST ABNORMAL AND/OR INCONCLUSIVE?
In cases where the results are not clear for one or more embryos or there are no unaffected embryos to transfer, thawing and doing another biopsy of those embryos might be an option.It is possible that an embryo that tests abnormal could result in a healthy child.
How to proceed if no embryos test normal is a complex decision and should be discussed before a cycle is started.
There are a lot of questions to think about, such as:
- How do you feel about the possibility of having an affected child?
- What do you hope to get out of the test?
- What types of abnormalities can be found?
- Are there other personal reasons that could affect your decision?)
- Are you willing to repeat a cycle if no embryos test normal?
- How many times?
You’re watching Additional Information, chapter six of the genetics and fertility video series from the American Society for Reproductive Medicine.
Transferring embryos that test normal may help lower the chance of miscarriage, but the risk is never zero.
But, it can also be stressful when you have a transfer of an embryo that tested normal and it doesn’t result in a pregnancy. IVF success can be affected by many other things that we don’t yet understand.
OTHER OPTIONS
For some families, another option is to conceive naturally, understanding the chance of having an affected child.For example, if both parents have an autosomal recessive single-gene defect for cystic fibrosis, you would have:
- a 1 in 4 chance to have a child with CF, and
- a 3 in 4 chance of having a healthy child.
- a 1 in 3 chance of a healthy child without the trait, and
- a 2 in 3 chance of having a child who is healthy but carries the trait like you do.
Some couples who do not want the invasiveness or expense of IVF and PGT could consider Intrauterine insemination–IUI also called artificial insemination. IUI is a medical procedure where prepared semen is injected into the biological mother’s uterus resulting in fertilization of her eggs. This is a less expensive, less invasive option.
Genetic Screening/Testing
Find a Health Professional